How To Determine Which Step Is The Slow Step In A Reaction
How To Determine Which Step Is The Slow Step In A Reaction. The rate determining step of a reaction is the slowest step in the mechanism. Now (or probably even before reaching this stage) you must write different rate equations (first.
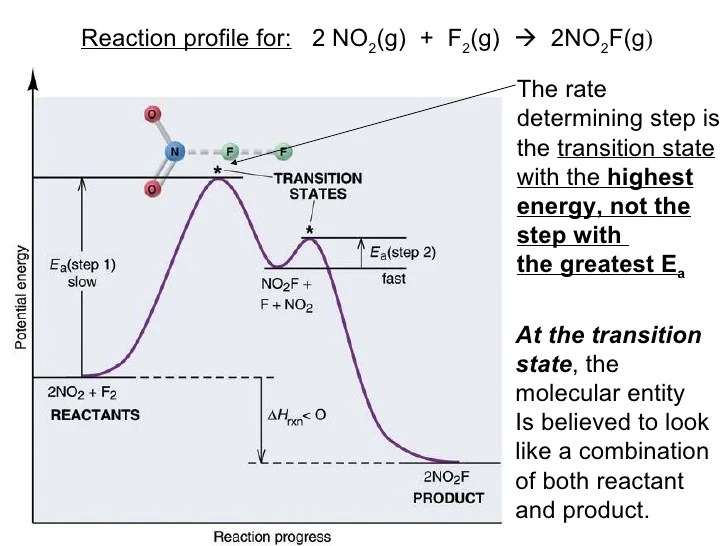
The rate determining step of a reaction is the slowest step in the mechanism. The rate determining step can be compared to. The two important components to look for when determining reaction mechanisms are 1) that the sum of elementary steps = overall reaction.
Reaction Rates & How To Determine Rate Law.
Now (or probably even before reaching this stage) you must write different rate equations (first. Many reaction mechanisms contain one step that is much slower than the others; The rate determining step of a reaction is the slowest step in the mechanism.
The Two Important Components To Look For When Determining Reaction Mechanisms Are 1) That The Sum Of Elementary Steps = Overall Reaction.
How to determine which step is the slow step in a reaction? The rate determining step is the slowest step of a chemical reaction that determines the speed (rate) at which the overall reaction proceeds. The rate determining step can be compared to.
Post a Comment for "How To Determine Which Step Is The Slow Step In A Reaction"